[00:00-00:01]: [EBGLYSS wave animates on screen; EBGLYSS waves whoosh through frame]
[00:02-00:04]: [EBGLYSS logo animates on]
[00:05-00:07]: [Woman is working on her computer]
[Legal Super] Actor portrayal.
[00:08-00:14]: [EBGLYSS logo and dosing information build and remain on the screen; title card builds on screen]
[VO] This video will show you how to properly administer EBGLYSS using the Prefilled Pen.
[Caption] Using your EBGLYSS Prefilled Pen (250 mg/2 mL)
[Legal Super] Please see safety information throughout this video and the Indication and Safety Summary at the end of the video.
[00:15-00:16]: [EBGLYSS logo and dosing information remain on the screen; title card builds on screen]
[VO] Please see safety information throughout this video…
[Caption] Using your EBGLYSS Prefilled Pen (250 mg/2 mL)
[Legal Super] Please see safety information throughout this video and the Indication and Safety Summary at the end of the video.
[00:17-00:19]: [EBGLYSS logo and dosing information remain on the screen; title card builds on screen]
[VO] …and the Indication and Safety Summary at the end of the video.
[Caption] Using your EBGLYSS Prefilled Pen (250 mg/2 mL)
[Legal Super] Please see safety information throughout this video and the Indication and Safety Summary at the end of the video.
[00:20-00:23]: [EBGLYSS logo and dosing information remain on the screen]
[VO] It is important to read the full Instructions for Use before injecting.
[Caption] Using your EBGLYSS Prefilled Pen (250 mg/2 mL)
[Legal Super] Read the full Instructions for Use before injecting.
[00:24-00:29]: [EBGLYSS logo and dosing information remain on the screen; injection chapters with icons build on screen; icons and corresponding copy animate on screen]
[VO] Do not use until you have been shown how to inject EBGLYSS by your healthcare provider.
[Caption] Get ready
Inject
Finish
[Legal Super] Do not use until you have been shown how to inject EBGLYSS by your healthcare provider. If you are a parent or caregiver giving an injection to someone else, be sure to read and understand the full Instructions for Use before injecting.
[00:30-00:32]: [EBGLYSS logo, dosing information, and injection chapters remain on screen; icons and corresponding copy animate on screen]
[VO] If you are a parent or caregiver giving an injection to someone else...
[Caption] Get ready
Inject
Finish
[Legal Super] Do not use until you have been shown how to inject EBGLYSS by your healthcare provider. If you are a parent or caregiver giving an injection to someone else, be sure to read and understand the full Instructions for Use before injecting.
[00:33-00:38]: [EBGLYSS logo, dosing information, and injection chapters remain on screen; icons and corresponding copy animate on screen]
[VO] …be sure to read and understand the full Instructions for Use before injecting.
[Caption] Get ready
Inject
Finish
[Legal Super] Do not use until you have been shown how to inject EBGLYSS by your healthcare provider. If you are a parent or caregiver giving an injection to someone else, be sure to read and understand the full Instructions for Use before injecting.
[00:39-00:45]: [Woman is working on computer and finishes writing an email]
[VO] Alright, now that work is in a good place, I'm ready for a walk…
[00:45-00:48]: [Woman receives a push alert notification on her phone (not showing the screen); calendar reminder appears on screen]
[VO] …and my next injection of EBGLYSS.
[Caption] Time for your next injection of EBGLYSS.
[00:49-00:50]: [Woman looks back at dog]
[VO] But before I go…
[00:51-00:52]: [Dog looks up from couch]
[VO] …I'll get my next injection of EBGLYSS…
[00:53-00:54]: [Woman interacts with dog and walks through room]
[VO] …ready for when I'm back.
[00:55-00:58]: [Woman and dog walk through doorframe into kitchen]
[VO] At first, I wasn't sure how EBGLYSS would fit into my life…
[00:59-01:01]: [Woman opens the fridge and takes EBGLYSS out; caption builds with fridge icon]
[VO] …but making EBGLYSS part of my routine was simple.
[Caption] Storing EBGLYSS
Store EBGLYSS in your refrigerator. Keep your Pen in the original carton to protect it from light. Keep away from children. Do not freeze. Do not shake.
[01:02-01:05]: [Woman opens the fridge and takes EBGLYSS out; caption builds with fridge icon]
[VO] Before I inject, I'll take EBGLYSS out of the fridge…
[Caption] Storing EBGLYSS
Store EBGLYSS in your refrigerator. Keep your Pen in the original carton to protect it from light.
Keep away from children. Do not freeze. Do not shake.
[01:06-01:09]: [Woman places EBGLYSS on the counter; caption builds with hourglass icon; 45-minute countdown builds]
[VO] …and place it on a flat surface so it can warm up to room temperature.
[Caption] Wait 45 minutes
With the gray base cap on, allow the Pen to warm up to room temperature for 45 minutes before injecting.
[Legal Super] Do not store above 86˚F (30˚C). Dispose of the EBGLYSS pen if not used within 7 days at room temperature. Do not warm up with a microwave, hot water, or sunlight. Do not use if medicine is frozen.
[01:10-01:12]: [Woman walks out of kitchen; hour glass icon and 45-minute countdown remain on screen]
[VO] I can also keep my Pen in the original carton at room temperature…
[Caption] Wait 45 minutes
With the gray base cap on, allow the Pen to warm up to room temperature for 45 minutes before injecting.
[Legal Super] Do not store above 86˚F (30˚C). Dispose of the EBGLYSS pen if not used within 7 days at room temperature. Do not warm up with a microwave, hot water, or sunlight. Do not use if medicine is frozen.
[01:13-01:16]: [Dog runs behind woman; 45-minute countdown begins]
[VO] …for up to 7 days, which is handy if I'm traveling.
[Legal Super] FOR CAREGIVERS
If you're a caregiver helping someone to inject, please carefully read the full Instructions for Use before injecting.
[01:17-01:19]: [Woman puts the leash on the dog and they walk out the front door; 45-minute countdown]
[VO] Ok boy, you ready? Let's go.
[Legal Super] FOR CAREGIVERS
If you're a caregiver helping someone to inject, please carefully read the full Instructions for Use before injecting.
[01:20-01:24]: [Woman is walking dog in park]
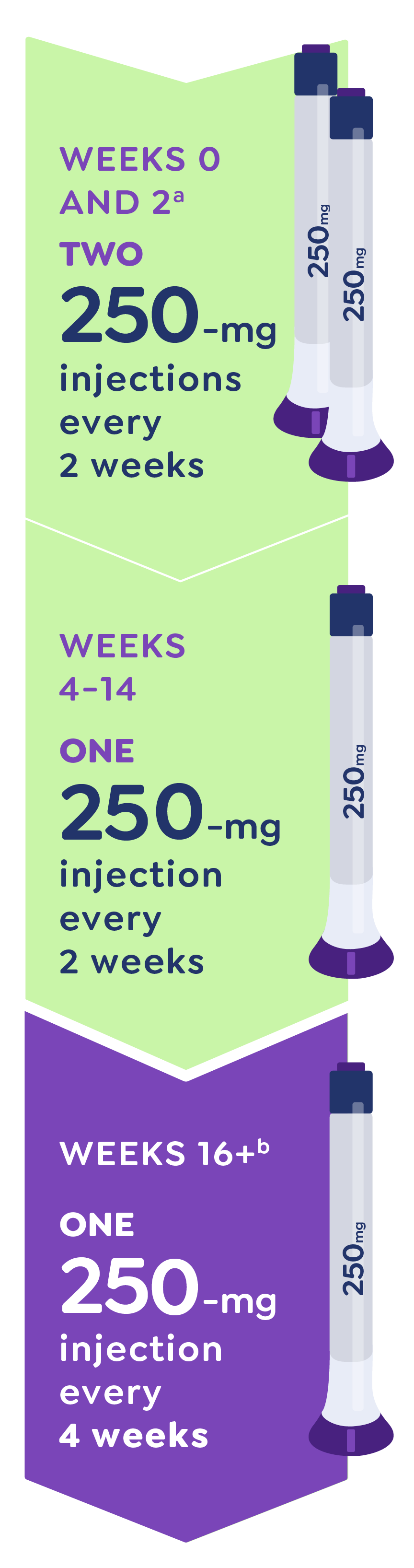
[VO] EBGLYSS is taken once every 2 weeks for 16 weeks…
[01:25-01:27]: [Dosing schedule animates over woman and dog walking in a park; dosing schedule animates]
[VO] …with 2 injections taken at week 0 and week 2.
[Legal Super] Please see the full dosing schedule and guidelines at:
https://EBGLYSS.lilly.com/taking-ebglyss[01:28-01:29]: [Dosing schedule continues animating over woman and dog walking in park; dosing schedule animates]
[VO] If you see skin improvements at 16 weeks…
[Legal Super] Please see the full dosing schedule and guidelines at:
https://EBGLYSS.lilly.com/taking-ebglyss[01:30-01:33]: [Dosing schedule continues animating over woman and dog walking in park; dosing schedule animates]
[VO] …you and your doctor can discuss switching to once-monthly dosing…
[Legal Super] Please see the full dosing schedule and guidelines at:
https://EBGLYSS.lilly.com/taking-ebglyss[01:34-01:36]: [Dosing schedule continues animating over woman and dog walking in park; dosing schedule animates]
[VO] …which is once every 4 weeks.
[Legal Super] Please see the full dosing schedule and guidelines at:
https://EBGLYSS.lilly.com/taking-ebglyss[1:37-1:41]: [Woman returns from walking the dog and hangs up the leash; 45-minute countdown comes to an end]
[VO] Alright, now it's time for my EBGLYSS injection.
[1:42-1:45]: [Woman enters the kitchen and stands next to where EBGLYSS has been resting in the carton; injection steps animate on screen]
[VO] At first, I was a little nervous about the injection process…
[Caption] 3 injection steps
Uncap the Pen
Place and Unlock
Press and Hold for 15 Seconds
[1:46-1:47]: [Woman inspects the EBGLYSS carton; injection steps animate on screen]
[VO] …but after learning these 3 basic steps…
[Caption] 3 injection steps
Uncap the Pen
Place and Unlock
Press and Hold for 15 Seconds
[1:48-1:49]: [Woman continues inspecting the EBGLYSS carton; injection steps remain on screen]
[VO] …I've really gotten the hang of it.
[Caption] 3 injection steps
Uncap the Pen
Place and Unlock
Press and Hold for 15 Seconds
[1:50-1:54]: [Woman removes EBGLYSS from the carton and inspects it; caption builds with magnifying glass icon]
[VO] Before I begin, I'll inspect the medicine to make sure it's not expired…
[1:55-1:57]: [Woman continues inspecting the EBGLYSS Pen; caption builds with magnifying glass icon]
[VO] …and the liquid is clear or colorless to slightly yellow.
[Caption] Inspect your Pen and medicine
√ Make sure you have the right medicine.
√ The medicine inside should be clear. It may be
colorless to slightly yellow to slightly brown.X Do not use if the Pen looks damaged, if the medicine is cloudy, is discolored, or has particles, or if the expiration date printed on the label has passed.
[1:58-1:59]: [Woman continues inspecting the EBGLYSS Pen; caption remains on screen]
[VO] Looks good to me.
[Caption] Inspect your Pen and medicine
√ Make sure you have the right medicine.
√ The medicine inside should be clear. It may be colorless to slightly yellow to slightly brown.
X Do not use if the Pen looks damaged, if the medicine is cloudy, is discolored, or has particles, or if the expiration date printed on the label has passed.
[2:00-2:02]: [Woman washes hands; caption builds with handwashing icon]
[VO] Next, I'll wash my hands with soap and water.
[Caption] Get ready
Wash your hands with soap and water.
[2:03-2:05]: [Woman walks over to counter; caption with handwashing icon remains on screen]
[VO] Now I'll choose my injection site.
[Caption] Get ready
Wash your hands with soap and water.
[2:06-2:08]: [Woman is choosing her injection site; caption builds with injection site icon; injection areas are highlighted on the body]
[VO] I can ask someone to help me inject in the back of my upper arm…
[Caption] Choose and clean your injection site
Do not inject in the exact same spot every time. You can use the same area of your body but choose a different spot in that area.
[2:09-2:13]: [Woman is choosing her injection site; caption remains on screen; injection areas are highlighted on the body]
[VO] …or I can inject myself in the front of my thigh or in my belly.
[Caption] Choose and clean your injection site
Do not inject in the exact same spot every time. You can use the same area of your body but choose a different spot in that area.
[2:14-2:16]: [Woman is choosing her injection site; caption remains on screen; injection areas are highlighted on the body]
[VO] Today, I will inject EBGLYSS in my belly.
[Caption] Choose and clean your injection site
Avoid areas where the skin is tender, bruised, red, hard, or in area of skin that is affected by atopic dermatitis or other skin lesions.
[2:17-2:20]: [Woman cleans belly with alcohol wipe; caption builds with check mark icon]
[VO] I'll clean the injection site with an alcohol wipe and let it dry.
[Caption] Prepare your skin
Clean your injection site with an alcohol wipe and let it dry.
[2:21-2:22]: [Woman ensures lock ring is locked; line circles the lock ring]
[VO] I'll make sure the Pen is locked.
[Legal Super] Needle End
The device in this video is for demonstration purpose only. The actual device will include the words Needle End near the location of the needle.
[2:23-2:26]: [Woman uncaps the Pen; caption builds with check mark icon]
[VO] Now I'll twist off the gray base cap and throw the cap away in my trash.
[Caption] Step 1
Uncap the Pen
[Legal Super] Needle End
Do not put the gray base cap back on–this could damage the needle. Do not touch the needle inside the clear base.
[2:27-2:30]: [Woman places the Pen against her body; caption builds with check mark icon]
[VO] I'll place and hold the clear base flat and firmly against my skin.
[Caption] Step 2
Place and Unlock
[2:31-2:35]: [Woman unlocks the pen; caption builds with check mark icon; circular line animates as the lock ring is unlocked]
[VO] With the clear base on my skin, I'll turn the lock ring to the unlock position.
[Caption] Step 2
Place and Unlock
[2:36-2:39]: [Woman hears the first click and holds the purple button for 15 seconds; caption builds with check mark icon; 15-second countdown builds]
[VO] All I need to do is press and hold the purple injection button…
[Caption] Step 3
Press and Hold for 15 Seconds
[2:40-2:43]: [Woman hears the first click and holds the purple button for 15 seconds; caption remains with check mark icon; 15-second countdown begins]
[VO] …for 15 seconds, until I hear two loud clicks.
[Caption] Step 3
Press and Hold for 15 Seconds
[2:44-2:47]: [Woman is holding the purple button for 15 seconds; caption remains with check mark icon; 15-second countdown continues]
[VO] The first click lets me know the injection has started.
[Caption] Step 3
Press and Hold for 15 Seconds
[2:48-2:50]: [Woman is holding the purple button for 15 seconds; caption remains with check mark icon; 15-second countdown continues]
[VO] It's nice that I don't have to see the needle.
[Caption] Step 3
Press and Hold for 15 Seconds
[2:51-2:52]: [Woman hears the 2nd click and releases the purple button; “Click” animates on Pen; 15-second countdown comes to an end]
[VO] I'll keep holding until I hear a second click…
[Caption] Step 3
Press and Hold for 15 Seconds
[2:53-2:55]: [Pen button is released; caption builds with star icon]
[VO] …which lets me know the injection is done.
[Caption] Finish
2 clicks, 15 seconds and the injection is finished. Another way to know the injection is complete is when the gray plunger is visible.
[2:56-3:00]: [Woman removes pen from injection site; caption remains on screen as gray plunger is highlighted]
[VO] And that's it! 2 clicks, 15 seconds, and I'm just about finished!
[Caption] Finish
2 clicks, 15 seconds and the injection is finished. Another way to know the injection is complete is when the gray plunger is visible.
[3:01-3:04]: [Woman prepares to dispose of EBGLYSS and pulls sharps disposal container from first shelf in the cabinet at eye level; caption builds with disposal icon]
[VO] After my injection, I'll safely throw away the used Pen…
[Caption] Dispose of used Pen properly
Put the used EBGLYSS Pen in an FDA-cleared sharps disposal container right away after use.
Do not throw away Pen(s) in your household trash.
[3:05-3:08]: [Woman disposes of EBGLYSS in sharps disposal container; Lilly Support Services logo; caption updates with new copy]
[VO] …in the free sharps disposal container that Lilly Support Services
™ provided.
[Caption] Dispose of used Pen properly
Keep sharps disposal container away from children and pets.
[3:09-3:10]: [Woman puts sharps disposal container back in cabinet; Lilly Support Services logo; caption updates with new copy]
[Caption] Dispose of used Pen properly
When your sharps disposal container is almost full, follow your community guidelines for the right way to throw it away. Do not recycle your used sharps disposal container.
[Legal Super] Visit
www.FDA.gov/safesharpsdisposal to lean about sharps disposal in your state.
[3:11-3:15]: [Woman gives dog a treat; Lilly Support Services logo; caption updates with new copy]
[VO] Don't worry boy, I didn't forget about you!
[Caption] Dispose of used Pen properly
When your sharps disposal container is almost full, follow your community guidelines for the right way to throw it away. Do not recycle your used sharps disposal container.
[Legal Super] Visit
www.FDA.gov/safesharpsdisposal to lean about sharps disposal in your state.
[3:16-3:17]: [Woman and dog walk back over to workstation where dog lies down next to desk; Lilly Support Services logo; caption builds with headset icon]
[VO] That didn't take long.
[Legal Super] Please carefully read the full Instructions for Use before injecting EBGLYSS.
[3:18-3:21]: [Woman pets dog; Lilly Support Services logo; caption builds with headset icon]
[VO] I'm glad that I can fit my monthly injection into my routine…
[Caption] Scan here
For additional support with virtual or over-the-phone training by a registered nurse.
[Legal Super] Please carefully read the full Instructions for Use before injecting EBGLYSS.
[3:22-3:24]: [Woman pets dog; Lilly Support Services logo; caption builds with headset icon]
[VO] …with only 3 basic steps.
[Caption] Scan here
For additional support with virtual or over-the-phone training by a registered nurse.
[Legal Super] Please carefully read the full Instructions for Use before injecting EBGLYSS.
[3:25-3:27]: [Woman returns to workstation]
[VO] Now, I can get back to my day.
[3:28-7:30]: [CBS scrolls]
[VO]
INDICATION AND SAFETY SUMMARYEBGLYSS is an injectable medicine used to treat adults and children 12 years of age and older who weigh at least 88 pounds (or 40 kg) with moderate-to-severe eczema, also called atopic dermatitis, that is not well controlled with prescription therapies used on the skin (or topical), or who cannot use topical therapies. EBGLYSS can be used with or without topical corticosteroids.
It is not known if EBGLYSS is safe and effective in children less than 12 years of age or in children 12 years to less than 18 years of age who weigh less than 88 pounds (or 40 kg).
WARNINGS - Do not use EBGLYSS if you are allergic to lebrikizumab-lbkz or to any of the ingredients in EBGLYSS. See the Patient Information leaflet that comes with EBGLYSS for a complete list of ingredients.
BEFORE USING
Before using EBGLYSS, tell your healthcare provider about all your medical conditions, including if you:Have a parasitic (or helminth) infection.
Are scheduled to receive any vaccinations. You should not receive a “live vaccine” if you are treated with EBGLYSS.
Are pregnant or plan to become pregnant. It is not known if EBGLYSS will harm your unborn baby. If you become pregnant during treatment with EBGLYSS, you or your healthcare provider can call Eli Lilly and Company at 1-800-LillyRx (1-800-545-5979) to report the pregnancy.
Are breastfeeding or plan to breastfeed. It is not known if EBGLYSS passes into your breast milk.
including prescription and over-the-counter medicines, vitamins, and herbal supplements.
POSSIBLE SIDE EFFECTSEBGLYSS can cause serious side effects, including:Allergic reactions. EBGLYSS can cause allergic reactions that may sometimes be severe. Stop using EBGLYSS and tell your healthcare provider or get emergency help right away if you get any of the following signs or symptoms:
breathing problems or wheezing
swelling of the face, lips, mouth, tongue or throat
hives
itching
fainting, dizziness, feeling lightheaded
skin rash
cramps in your stomach area (or abdomen)
Eye problems. Tell your healthcare provider if you have any new or worsening eye problems, including eye pain or changes in vision, such as blurred vision
The most common side effects of EBGLYSS include:
These are not all of the possible side effects of EBGLYSS. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or
www.fda.gov/medwatch.
HOW TO TAKE
See the detailed “Instructions for Use” that comes with EBGLYSS for information about how to prepare and inject EBGLYSS and how to properly store and throw away (or dispose of) used EBGLYSS prefilled pens and prefilled syringes.
Use EBGLYSS exactly as prescribed by your healthcare provider.
EBGLYSS is given as an injection under the skin (or subcutaneous injection)
If your healthcare provider decides that you or a caregiver can give the injections of EBGLYSS, you or a caregiver should receive training on the right way to prepare and inject EBGLYSS. Do not try to inject EBGLYSS until you have been shown the right way by your healthcare provider. In children 12 years of age and older, EBGLYSS should be given by a caregiver.
If you miss a dose of EBGLYSS, inject the missed dose as soon as possible, then inject your next dose at your regular scheduled time.
EBGLYSS is a prescription medicine available as a 250 mg per 2 mL injection prefilled pen or prefilled syringe. For more information, call
1-800-545-5979 or go to
ebglyss.lilly.comThis summary provides basic information about EBGLYSS but does not include all information known about this medicine. Read the information that comes with your prescription each time your prescription is filled. This information does not take the place of talking to your doctor. Be sure to talk to your doctor or other healthcare provider about EBGLYSS and how to take it. Your doctor is the best person to help you decide if EBGLYSS is right for you.
[Caption] INDICATION AND SAFETY SUMMARYEBGLYSS
™ (EHB-glihs) is an injectable medicine used to treat adults and children 12 years of age and older who weigh at least 88 pounds (40 kg) with moderate-to-severe eczema (atopic dermatitis) that is not well controlled with prescription therapies used on the skin (topical), or who cannot use topical therapies. EBGLYSS can be used with or without topical corticosteroids.
It is not known if EBGLYSS is safe and effective in children less than 12 years of age or in children 12 years to less than 18 years of age who weigh less than 88 pounds (40 kg).
WARNINGS - Do not use EBGLYSS if you are allergic to lebrikizumab-lbkz or to any of the ingredients in EBGLYSS. See the Patient Information leaflet that comes with EBGLYSS for a complete list of ingredients.
BEFORE USINGBefore using EBGLYSS, tell your healthcare provider about all your medical conditions, including if you:
Have a parasitic (helminth) infection.
Are scheduled to receive any vaccinations. You should not receive a “live vaccine” if you are treated with EBGLYSS.
Are pregnant or plan to become pregnant. It is not known if EBGLYSS will harm your unborn baby. If you become pregnant during treatment with EBGLYSS, you or your healthcare provider can call Eli Lilly and Company at 1-800-LillyRx (1-800-545-5979) to report the pregnancy.
Are breastfeeding or plan to breastfeed. It is not known if EBGLYSS passes into your breast milk.
including prescription and over-the-counter medicines, vitamins, and herbal supplements.
POSSIBLE SIDE EFFECTSEBGLYSS can cause serious side effects, including:Allergic reactions. EBGLYSS can cause allergic reactions that may sometimes be severe. Stop using EBGLYSS and tell your healthcare provider or get emergency help right away if you get any of the following signs or symptoms:
breathing problems or wheezing
swelling of the face, lips, mouth, tongue or throat
hives
itching
fainting, dizziness, feeling lightheaded
skin rash
cramps in your stomach area (abdomen)
Eye problems. Tell your healthcare provider if you have any new or worsening eye problems, including eye pain or changes in vision, such as blurred vision.
The most common side effects of EBGLYSS include:
These are not all of the possible side effects of EBGLYSS. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or
www.fda.gov/medwatch.
HOW TO TAKE
See the detailed “Instructions for Use” that comes with EBGLYSS for information about how to prepare and inject EBGLYSS and how to properly store and throw away (dispose of) used EBGLYSS prefilled pens and prefilled syringes.
Use EBGLYSS exactly as prescribed by your healthcare provider.
EBGLYSS is given as an injection under the skin (subcutaneous injection).
If your healthcare provider decides that you or a caregiver can give the injections of EBGLYSS, you or a caregiver should receive training on the right way to prepare and inject EBGLYSS. Do not try to inject EBGLYSS until you have been shown the right way by your healthcare provider. In children 12 years of age and older, EBGLYSS should be given by a caregiver.
If you miss a dose of EBGLYSS, inject the missed dose as soon as possible, then inject your next dose at your regular scheduled time.
EBGLYSS is a prescription medicine available as a 250 mg/2 mL injection prefilled pen or prefilled syringe. For more information, call
1-800-545-5979 or go to
ebglyss.lilly.comThis summary provides basic information about EBGLYSS but does not include all information known about this medicine. Read the information that comes with your prescription each time your prescription is filled. This information does not take the place of talking to your doctor. Be sure to talk to your doctor or other healthcare provider about EBGLYSS and how to take it. Your doctor is the best person to help you decide if EBGLYSS is right for you.
LK CON BS AD APP
EBGLYSS
™, its delivery device base, and Lilly Support Services
™ are trademarks owned or licensed by Eli Lilly and Company, its subsidiaries, or affiliates. PP-LK-US-0005 09/2024 © Lilly USA, LLC 2024. All rights reserved.
[7:31-7:32]: [EBGLYSS waves animate on screen; EBGLYSS waves whoosh through frame]
[7:33-7:35]: [EBGLYSS logo animates on]